-
近年来,TMCs半导体材料由于其突出的物理、化学特性而受到越来越多的关注[1]。作为TMCs家族中的重要成员,CoS具有稳定的结构,且毒性比传统半导体材料低[2]。CoS晶体属六方晶系,Co-S键长为2.32 Å (1 Å=0.1 nm),空间群为P63/mmc (194),有着良好的对称性。由于CoS QDs禁带宽度较窄因而具有优异的近红外吸收特性,引起了人们的研究兴趣,并将其应用于光催化、太阳电池、激光器等领域。Joshi等[3]采用微波辐射法合成了平均粒径为58.28 nm,带隙为1.69 eV的CoS纳米颗粒(CoS NPs),该颗粒在200~2 000 nm波长范围内具有较强的吸收特性;Rani等[4]采用水热法合成了粒径为22 nm的CoS NPs,通过紫外-可见(UV-Vis)和PL测试发现当激发波长为330 nm时,该CoS NPs在360~580 nm波长范围内有发光现象,且在近红外区域具有吸收特性;Pourahmad等[5]先通过水热法合成了具有近红外吸收特性的CoS NPs,其平均尺寸约为58 nm,然后再采用离子交换法制备了CoS/铝硅酸盐纳米光催化剂,研究结果表明:当CoS的质量分数为17%时,该光催化剂对亚甲基蓝的降解效率最高; Kumar等[6]采用水热法合成得到了带隙为1.27 eV,具有八角结构的花形CoS纳米材料,UV-Vis测试发现该材料在可见光及近红外区域均有较宽的吸收,基于该CoS制备的对电极可提高染料敏化太阳电池的电流密度;Kung等[7]通过两步法在掺氟氧化锡衬底上制备了一维CoS针状纳米棒阵列,研究表明该CoS纳米棒阵列具有优异的近红外吸收特性,可用于制作染料敏化太阳电池的对电极,在100 mW/cm2光照下,CoS针状纳米棒阵列电池的最大功率转换效率为7.67%;Hui等[8]利用液相剥离法制备得到厚度为2 nm的CoS 纳米片,在200~1900 nm波长范围内具有宽带吸收,并基于该纳米片制备了调制深度为6.4%的新型CoS 纳米片可饱和吸收体,成功应用于锁模光纤激光器。
为进一步提升CoS纳米材料的光学特性和加工性能,CoS纳米薄膜及CoS纳米复合物的研究得到了广泛关注。Ighodalo等[9]采用化学沉积法成功制备得到了具有不同晶粒尺寸的CoS纳米薄膜,经研究发现这些薄膜的直接带隙在1.9~2.2 eV之间,平均光导率为1.77×1017 S,随着沉积循环次数的增加,CoS纳米薄膜在近红外区域的吸收逐渐增强,但薄膜的结晶度却逐渐降低,在大约50次沉积循环后,薄膜呈现出非晶态。Li等[10]将所制备的CoS纳米片与聚乙二醇(PEG)混合得到了CoS-PEG纳米片,该复合材料具有强烈的近红外吸收特性和优异的光热稳定性; Ding[11]等通过原位还原硫化/碳化2-甲基咪唑钴盐(ZIF-67)制备了粒径小于5 nm的CoS QDs,然后与氮掺杂的碳纳米管(CNTs)结合形成了CoS QDs/CNTs复合材料,UV-Vis测试表明该复合材料在200~800 nm波长范围内有吸收,表现出优异的近红外特性,且在短时间内可实现对活性氧的100%降解。
在不同CoS纳米材料中,CoS QDs因禁带宽度较窄、量子尺寸效应显著,具有载流子浓度高、光吸收系数大等特性,应用前景广泛。为了减少量子点应用过程中的聚集,将其与有机聚合物复配能够有效提升量子点的稳定性及加工性能,利于获得高效稳定的发光器件[12]。聚二甲基硅氧烷(PDMS)是一种具有高透明性、高柔韧性及高安全性的有机聚合物,常用作复合材料的配体。液相超声剥离法是制备纳米材料的一类常见方法,其操作简便、成本低廉、安全可控。因此,文中采用液相超声剥离法制备了CoS QDs,再通过共混法将CoS QDs与 PDMS复配得到CoS QDs/PDMS纳米复合薄膜,并对CoS QDs和CoS QDs/PDMS纳米复合薄膜的红外光学特性进行了研究,以期拓展CoS纳米复合薄膜材料在红外光学领域的应用。
-
实验采用液相超声剥离法制备CoS QDs溶液,制备步骤如下:称取0.15 g CoS粉末(纯度≥99.5%)放至研钵中充分研磨2 h;将研磨后的CoS粉末与50 mL无水乙醇(纯度≥99.7%)分散剂混合均匀,并将其置于超声仪中以90 W的功率累计超声2 h;将超声后的溶液进行离心,转速500 r/min,时间5 min;取出上层清液,即得到CoS QDs溶液;将CoS QDs溶液进行干燥处理备用。
-
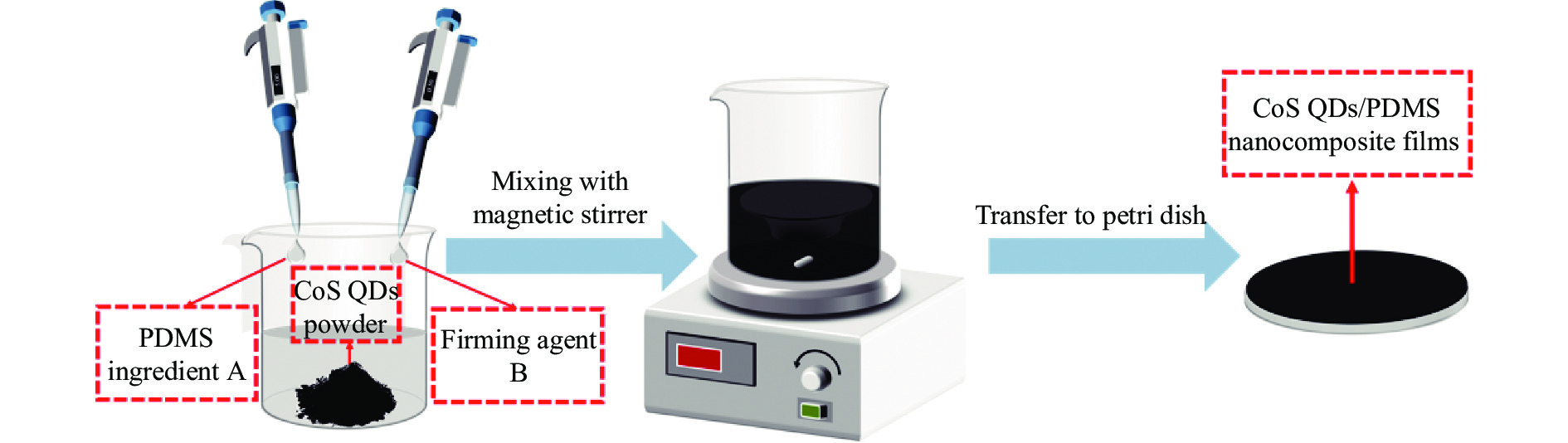
实验采用共混法制备CoS QDs/PDMS纳米复合薄膜,制备步骤如图1所示。取5 mL PDMS的基本组分 A 和0.5 ml固化剂 B 移至烧杯中,并加入0.05 g 经干燥处理的CoS QDs粉末,使用磁力搅拌器搅5 min,随后转移至培养皿中,以30 ℃加热干燥直至成膜。
-
使用透射电镜(TEM, Tecnai G2 TF30 S-Twin)、原子力显微镜(AFM, 日本精工SPA-400)以及能谱仪 (EDS, NOVA NANOSEM 450)对CoS QDs的尺寸、形貌、结构及元素组分进行表征;使用X射线光电子能谱 (XPS, PHI Versa 探针 II)、X射线衍射仪(XRD, EMPYREAN, X射线源:Cu Ka,λ=0.154178 nm)、傅里叶变换红外光谱仪 (FTIR, Nicolet iS10)和拉曼光谱仪(Raman Renishaw-InVia)对CoS QDs的物相组成及成键特性进行分析;使用紫外-可见分光光度计 (UV-Vis, Shimadzu UV-3600 Puls)和荧光光谱仪 (PL&PLE, Hitachi, F-4500)对CoS QDs和CoS QDs/PDMS纳米复合薄膜的光学性质进行测试。
-
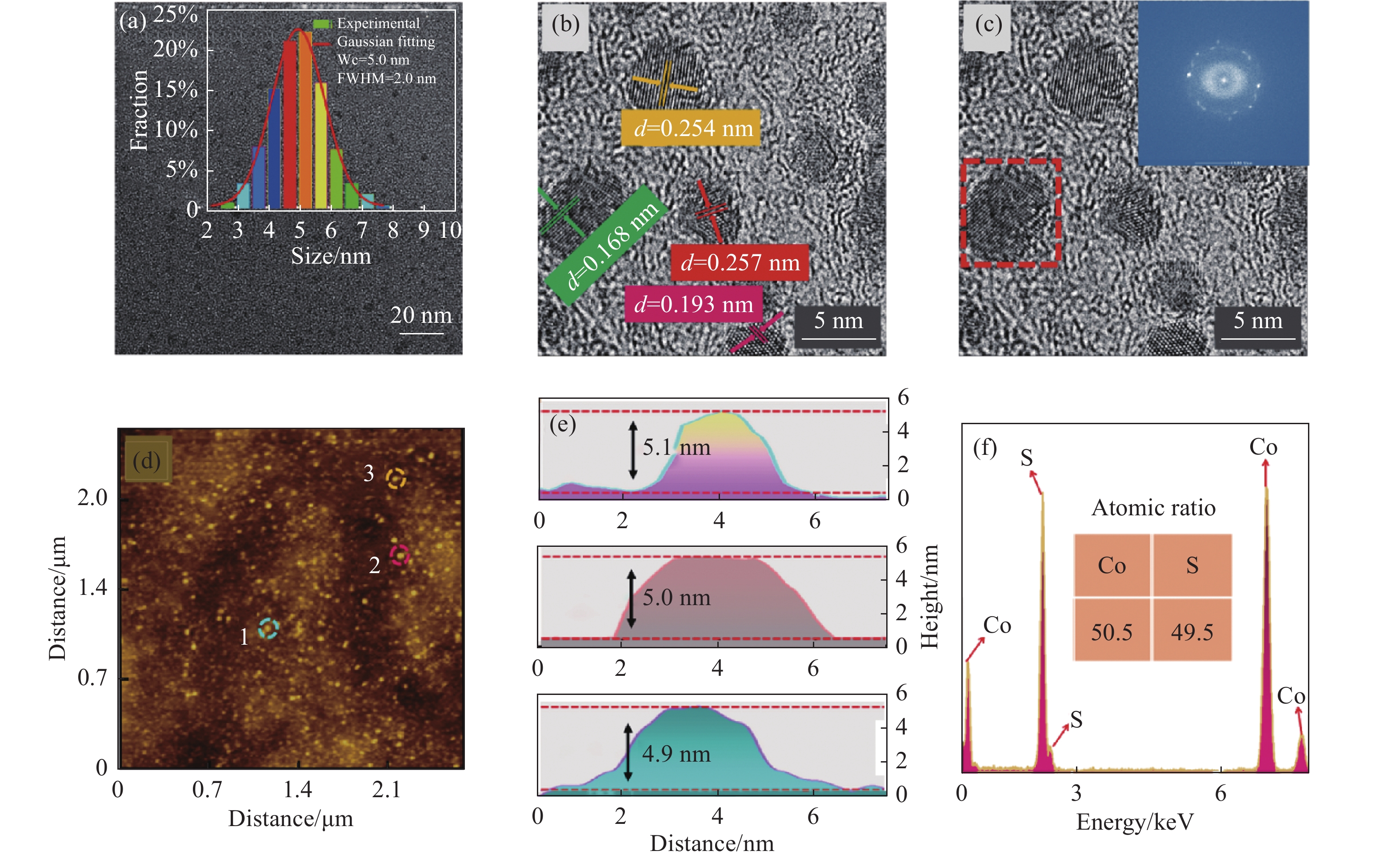
图2(a)为CoS QDs的TEM图和粒径分布直方图,可以看出CoS QDs具有良好的分散性,粒径符合正态分布,高斯拟合得到CoS QDs平均粒径约为5 nm,半峰宽(FWHM)为2 nm;图2(b)为CoS QDs的高分辨率TEM图,对其中的量子点使用Line Profile进行分析,得出晶格间距d=0.168 nm、d=0.193 nm、d=0.254 nm和d=0.257 nm,并分别对应于(1 1 0)、(1 0 2)、(1 0 1)和(0 0 2)晶面;图2(c)为在CoS QDs的高分辨率TEM图中选取其中一个量子点进行快速傅里叶变换分析(FFT),观察到CoS QDs呈现六方晶型,表明CoS QDs与CoS体材料的晶体结构相同。图2(d)为CoS QDs的AFM测试图,可以观察到CoS QDs尺寸均一、分布均匀,从中随机选取3个量子点,并标记为1、2、3进行粒径分析得到高度分别为4.9 nm、5.0 nm和5.1 nm,如图2(e)所示,该结果与TEM的粒径分析相吻合,表明CoS QDs呈球形。图2(f)为CoS QDs的EDS元素分析图,EDS能谱用于定性分析CoS QDs中元素的相对含量,在去除测试时包含的Cu、C等元素的干扰后得到Co元素(50.5%)与S元素(49.5%)的原子比例接近1∶1,符合CoS的1个Co原子与1个S原子相结合的结构模型。
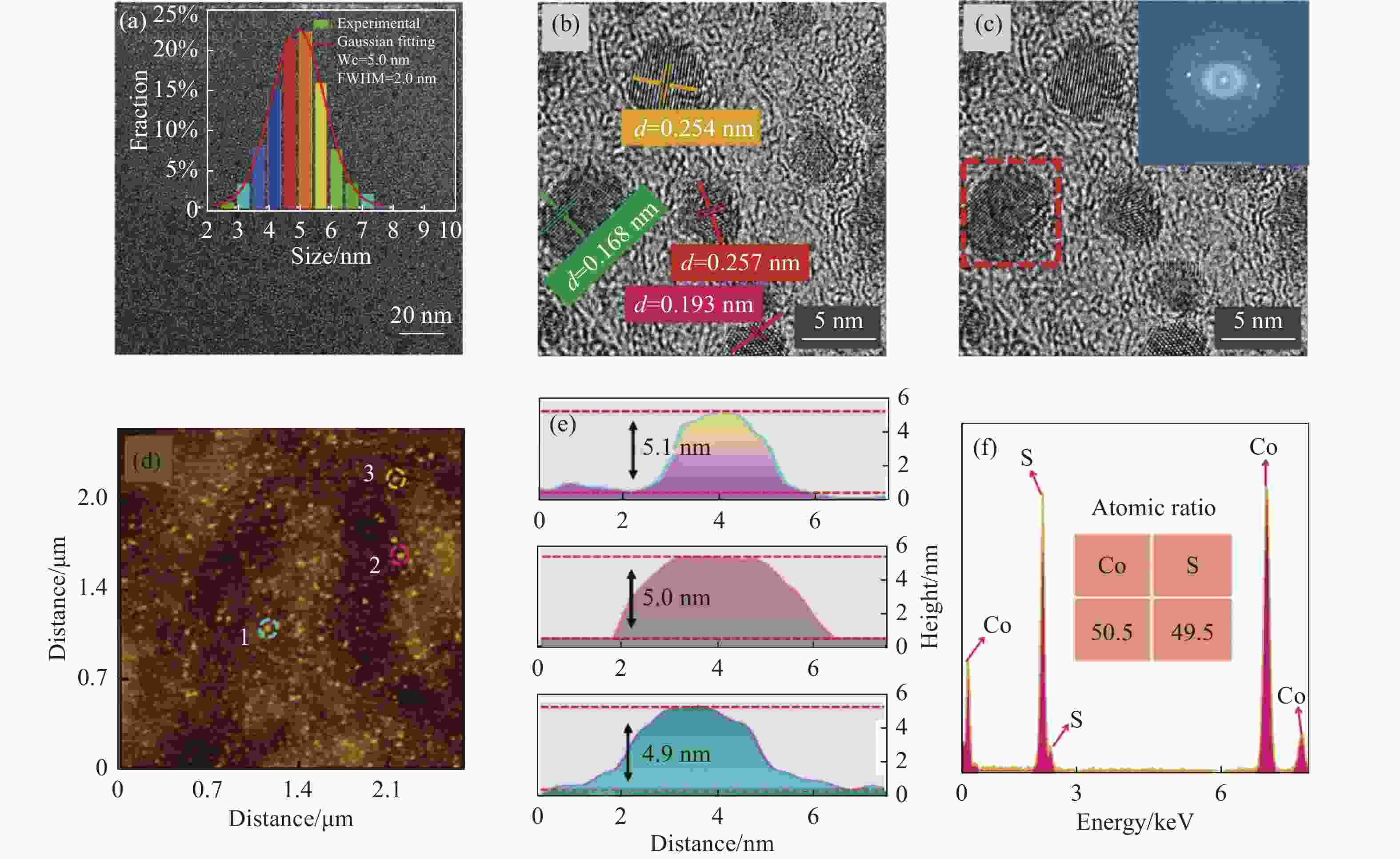
Figure 2. The morphology and component analysis of CoS QDs. (a) TEM image (inset shows the particle size distribution); (b) HR-TEM image; (c) HR-TEM image (inset shows the FFT pattern); (d) AFM image; (e) Height analysis of the particle size at positions 1, 2 and 3 marked in Fig.(d); (f) EDS energy spectrum of CoS QDs
-
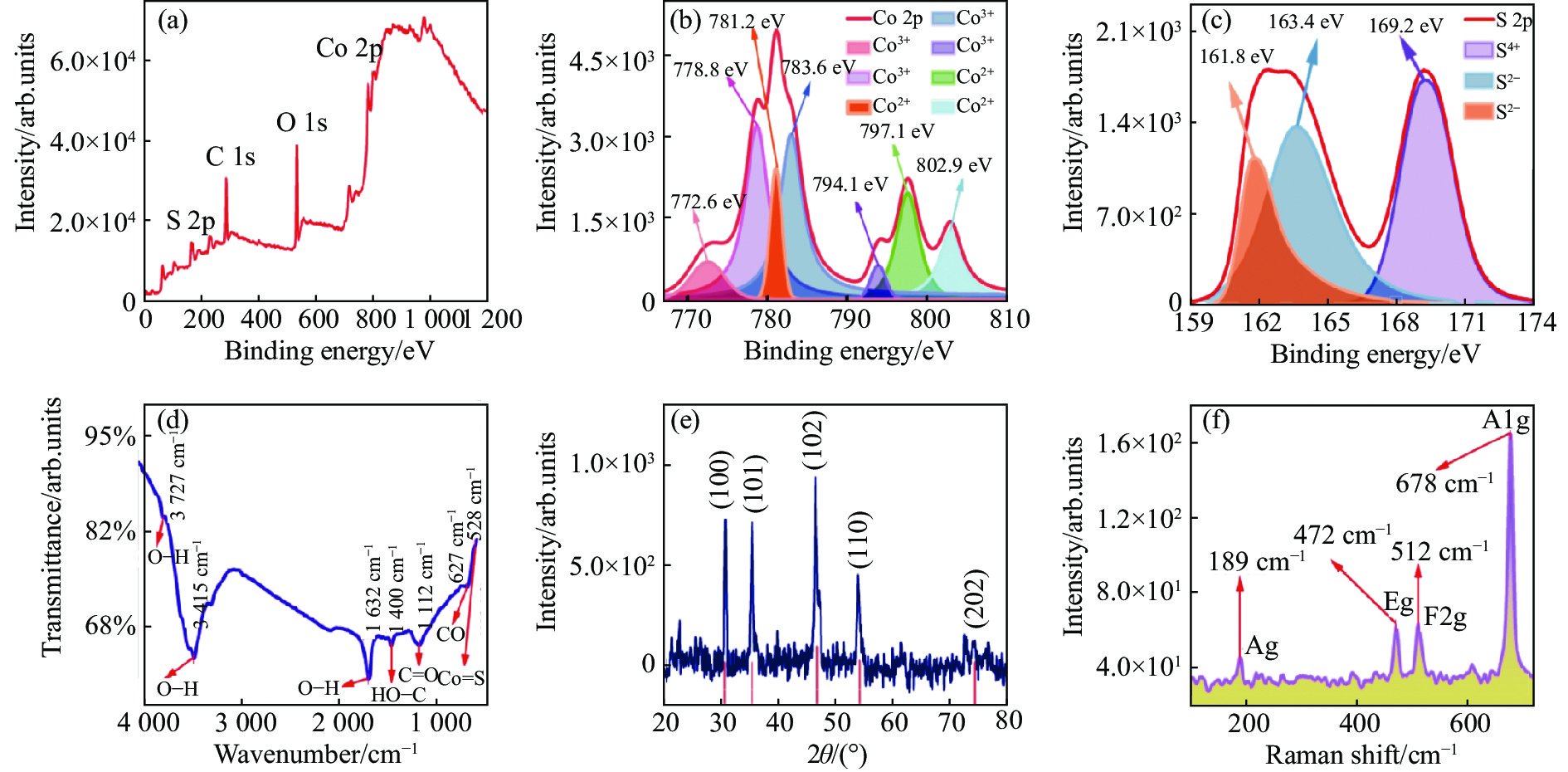
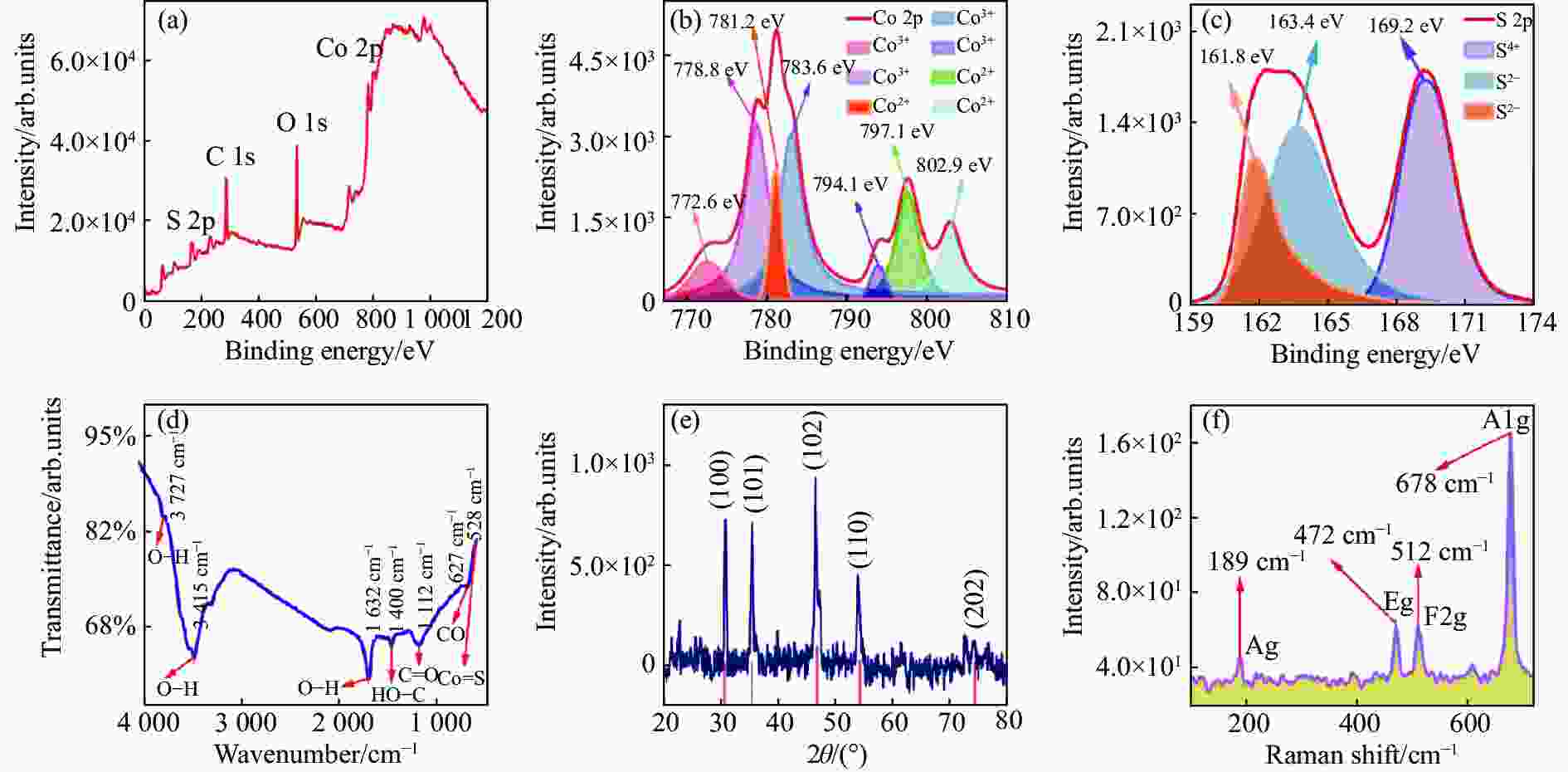
图3(a)为CoS QDs的XPS全谱图,从图中可以清晰地看到Co 2p、S 2p、C 1s和O 1s的XPS峰,其中Co 2p和S 2p是CoS的特征峰,C 1s可能是无水乙醇分散剂的残余,O 1s可能是样品CoS QDs表面被氧化或是无水乙醇分散剂的残余;为了深入分析CoS QDs中Co 2p和S 2p的存在形式,进行XPS分峰拟合。Co 2p的XPS谱如图3(b)所示,共有7种类型的峰,分别为Co 2p3/2(772.6 eV)、Co 2p3/2(778.8 eV)、Co 2p3/2(781.2 eV)、Co 2p3/2(783.6 eV)、Co 2p1/2(794.1 eV)、Co 2p1/2(797.1 eV)和Co 2p1/2(802.9 eV),其中前面6个峰都属于特征峰,772.6 eV、778.8 eV、794.1 eV对应Co3+[13], 781.2 eV、783.6 eV和797.1 eV对应Co2+[14],而802.9 eV对应的峰为卫星峰[15-16]。S 2p的XPS谱如图3(c)所示,共有3种类型的峰,分别为S 2p3/2(161.8 eV)、S 2p1/2(163.4 eV)和S 2p3/2(169.2 eV),其中161.8 eV和163.4 eV处出现的峰表明S是以S2−存在[17-19],而169.2 eV处出现的峰表明有S-O键存在[20],这可能是CoS表面的硫被氧化所导致的[13]。
FTIR测试结果如图3(d)所示,在528 cm−1和627 cm−1处分别观察到Co-S 键和CoS表面钴原子伸缩振动峰[21],其他振动为无水乙醇或无水乙醇氧化后所含的C、H和O所结合的化学键。CoS QDs的XRD测试结果如图3(e)所示,经过与标准PDF卡片(PDF#75-0605)对比可知,图中2θ=30.542° (d=0.29246 nm)、2θ=35.262°(d=0.25431 nm)、2θ=46.977° (d=0.19326 nm)、2θ=54.283° (d=0.16885 nm) 和 2θ=74.569° (d=0.12716 nm)处的衍射峰分别对应于CoS样品的(1 0 0)、(1 0 1)、(1 0 2)、(1 1 0)和(2 0 2)晶面,图中没有出现其他衍射峰,表明样品纯度高;CoS QDs的Raman测试结果如图3(f)所示,可以看到有4个清晰的Raman特征峰:189 cm−1、472 cm−1、512 cm−1和678 cm−1,分别对应硫化钴体材料的Ag、Eg、F2g和A1g振动模式[14,22-24],表明CoS是切实存在的。
-
CoS QDs溶液的UV-Vis吸收光谱测试结果如图4(a)所示,该图表明CoS QDs从紫外到红外波段(200~2 000 nm)都有较强的吸收,且样品在相同浓度下吸收强度基本保持不变,随着样品稀释倍数的增加吸收强度逐渐降低。图4(a)中的插图则表明CoS QDs溶液具有荧光效应:样品在自然光照射下呈现无色,在波长为254 nm和356 nm的紫外光照射下,分别呈现出淡紫色和蓝色。为了检测纯CoS QDs薄膜的吸收特性,以石英为衬底将 CoS QDs 溶液制备成膜进行测试(图4(b)),由此可以看出,CoS QDs薄膜在紫外到红外波段(200~2 000 nm)同样具有吸收,但整个区间的吸收强度随波长增加呈下降趋势。CoS QDs/PDMS纳米复合薄膜的UV-Vis吸收光谱测试结果如图4(c)所示,插图是自然光照下的PDMS 薄膜(左)和 CoS QDs/PDMS纳米复合薄膜(右)的照片。图中下方的橙色曲线是PDMS薄膜的吸收,可以看到PDMS薄膜本身在200-2200 nm波长范围内基本没有吸收;红色曲线为CoS QDs/PDMS纳米复合薄膜的UV-Vis吸收,可以看到该纳米复合薄膜从紫外到红外波段(200~2200 nm)都具有强烈吸收, 且整个区间的吸收强度随波长的增加而缓慢增加,表明CoS QDs与PDMS复合成膜以后,红外吸收特性得到有效增强。这是因为复合薄膜中的CoS QDs浓度较高,从而表现出较强的红外吸收特性;图中蓝色曲线表示放置六个月的CoS QDs/PDMS纳米复合薄膜吸收曲线,可以看出经过长时间放置的薄膜其UV-Vis吸收强度几乎没有发生变化,光学性质十分稳定。
图4(d)是CoS QDs的PL光谱图,从图中可以看到,当激发光波长从360 nm增加到500 nm (步长为20 nm)时,CoS QDs的PL峰逐渐红移。经归一化处理的PL光谱如图4(e)所示,该图更直观地反映了这一红移现象,其PL峰值所对应的波长变化为:506.4 nm→517.6 nm→522 nm→523.6 nm→528.4 nm→533.4 nm→539.2 nm→545.6 nm,通过CoS QDs的PL峰红移可以看出激发波长会直接影响CoS QDs的光致发光。图4(f)是CoS QDs在近红外波段的PL光谱图,当激发波长以50 nm的步长从550 nm增加至750 nm时,从图4(f) 内嵌的放大图可以清晰地看到CoS QDs在近红外波段仍存在发光现象。图4(g)是CoS QDs的光致发光激发(PLE)光谱图,从图中可以看到,当发射光波长在420-550 nm范围内依次增加时,CoS QDs的PLE峰也出现类似PL峰的红移现象。归一化处理的PLE光谱图如图4(h)所示,其PLE峰值所对应的波长变化为:368.4 nm→373.6 nm→380 nm→393.6 nm→396.2 nm→406.2 nm;图4(i)是CoS QDs在近红外波段的PLE光谱图,当发射光波长从600 nm增加至750 nm时(步长为50 nm),从图4(i)中的插图可以看到CoS QDs在近红外波段仍存在响应。通过上述PL和PLE光谱分析可知CoS QDs溶液的发光具有波长依赖性,并且存在明显的Stokes位移效应。

Figure 4. Infrared characterization of CoS QDs and their PDMS nanocomposite films. (a) UV-Vis absorption spectra of CoS QDs solutions (inset: photos of CoS QDs solutions under natural and UV light); (b) UV-Vis absorption spectra of CoS QDs films; (c) UV-Vis absorption spectra of CoS QDs of PDMS and CoS QDs/PDMS nanocomposite films; (d) PL spectra of CoS QDs solution; (e) PL spectra of normalized CoS QDs; (f) PL spectra of NIR CoS QDs; (g) PLE spectra of CoS QDs; (h) PLE spectra of CoS QDs; (i) PLE spectra of NIR CoS QDs; (j) PL spectra of PDMS plots; (k) PL spectra of CoS QDs/PDMS nanocomposite films; (l) PL spectra of near-infrared CoS QDs/PDMS nanocomposite films
图4(j)和(k)分别是PDMS薄膜和CoS QDs/PDMS纳米复合薄膜的PL光谱图,从图4(j)中可以看出,当激发光波长从320 nm逐渐增加到400 nm (步长20 nm)时,PDMS薄膜仅现了微弱的PL峰,并且随着激发波长的增加PDMS薄膜的发光强度逐渐降低;而图4(k)中的CoS QDs/PDMS纳米复合薄膜则具有明显的发光峰,说明在相同的波长激发下CoS QDs/PDMS纳米复合薄膜获得的能量更多,且出现的PL峰呈现出明显的红移现象。但与CoS QDs溶液相比,纳米复合薄膜的发光强度有所减弱,这可能是因为复合薄膜中的CoS QD浓度较高发生了浓度淬灭,从而导致纳米复合薄膜发光强度降低。图4(l)是CoS QDs/PDMS纳米复合薄膜在近红外波段的 PL 图,当激发光波长以50 nm的步长从600 nm增加至750 nm时,从图4(l)的插图可以看到CoS QDs/PDMS纳米复合薄膜在近红外波段(620-900 nm)仍具有发光现象。
-
文中通过液相超声剥离法成功制备出了具有分散性良好、颗粒大小均匀、平均粒径约为5 nm的球形CoS QDs,并采用共混法将CoS QDs与PDMS混合制备出了CoS QDs/PDMS纳米复合薄膜;通过UV-Vis测试发现CoS QDs溶液从紫外到红外波段(200~2 000 nm)都有吸收,且吸收强度随样品浓度的降低而降低;CoS QDs/PDMS纳米复合薄膜从紫外到红外波段(200~2200 nm)都具有吸收,与CoS QDs薄膜相比,CoS QDs/PDMS纳米复合薄膜的红外吸收特性得到有效增强,并且薄膜样品放置六个月后吸收强度几乎没有发生变化;通过PL测试发现CoS QDs和CoS QDs/PDMS纳米复合薄膜在红外波段均具有光致发光现象,PL峰有明显红移现象,存在 Stokes 位移效应,两者的发光都具有波长依赖性。综上,基于CoS QDs制备的CoS QDs/PDMS纳米复合薄膜具有优异的光学特性,尤其是红外波段的吸收和发光特性尤为瞩目,且光学性质稳定,表明该复合材料在红外探测器、纳米光子器件、柔性显示器、红外激光器等领域具有重要的潜在应用价值,值得进一步探索和研究。
Study on preparation and infrared properties of CoS QDs/PDMS nanocomposite films
doi: 10.3788/IRLA20230393
- Received Date: 2023-07-02
- Rev Recd Date: 2023-08-05
- Available Online: 2023-12-22
- Publish Date: 2023-12-22
-
Key words:
- liquid phase ultrasonic exfoliation /
- cobalt sulfide /
- quantum dots /
- nanocomposite film /
- infrared characteristic
Abstract:









 DownLoad:
DownLoad: