-
过渡金属硫属化合物(Transition metal chalcogenides, TMCs)是一类具有特殊结构的层状化合物,由过渡金属和硫族元素组成,因其具有较高的化学稳定性、电子传输能力及生物相容性,被广泛应用于光电器件、催化、生物医学等领域[1-4]。硫化亚铁(FeS)是一种典型的TMCs,具有密排六方晶体结构,属于P63/mmc空间群,对称性良好。FeS体材料具有良好导电性、磁性和光催化特性,其纳米材料光电性能优异,化学性质稳定,生物相容性好,在光电子器件、磁性材料及生物医学等领域具有广泛的应用前景。Maji等人[5]利用化学沉积法(CBD)在涂有氟掺杂氧化锡(FTO)的玻璃基底上沉积FeS纳米晶薄膜,将其作为光阳极构建光电化学太阳能电池,转换效率为2.6%,展现出FeS纳米薄膜在太阳电池领域的应用潜力;Malek等人[6]以硝酸铁和硫代硫酸盐为前驱体,分别通过CBD和旋涂沉积法制备得到两种FeS薄膜,结果表明:两种薄膜在可见光到近红外波长范围内均有稳定的吸收特性,其直接光学带隙分别为1.75 eV(CBD)和1.81 eV(旋涂);Yang等人[7]经过高温化学法合成FeS纳米片,利用聚乙二醇(PEG)进行功能化得到FeS-PEG,研究发现,FeS-PEG在近红外波段具有较强的吸收能力,并表现出强超顺磁性,可用于实现肿瘤高效光热消融。近年来,FeS量子点(quantum dots, QDs)因具有高的比表面积、良好的红外吸收特性及生物低毒性等优势备受瞩目。Yang等人[8]将白蛋白(BSA)作为约束剂,采用仿生法成功合成了FeS@BSA QDs,平均尺寸约3 nm,在近红外区域表现出强吸收特性,可进一步用于肿瘤的光热治疗。
目前,FeS纳米材料的研究主要集中于FeS纳米薄膜和纳米颗粒,有关FeS QDs及其复合材料的研究报道相对较少。随着光电器件、生物成像等领域对新型量子点材料的需求不断增加,加快相关的研究工作是很必要的。液相超声剥离是一种常见的材料制备方法,早期多用于层状材料的剥离:在液相环境中,辅助剂在超声波的驱动下进入到材料分子内部,借助超声波脉冲能量破环层间作用力,使片状结构逐渐剥离,形成片层极少的纳米材料。该方法设备简单、耗材少、容易操控,现已发展为一种制备QDs的方法。
聚乙烯醇(PVA)是一种水溶性聚合物,具有透明度高、热稳定性好、绿色无毒及成膜能力强等优点,可作为基底材料与FeS QDs复配并提供柔性支撑,同时可以有效减少FeS QDs团聚,提升其稳定性和加工性能。文中采用工艺简便、成本低廉的液相超声剥离法制备FeS QDs,并将FeS QDs与PVA复配得到FeS QDs/PVA纳米复合薄膜,对制备得到的FeS QDs及其PVA纳米复合薄膜进行红外光学性能研究,以期发现FeS QDs及其复合薄膜在红外光学领域的更多应用潜力。
-
实验采用液相超声剥离法制备FeS QDs,制备流程如图1所示。称取0.15 g FeS粉末(纯度≥99.9%)置于研钵中研磨2 h;将磨好的粉末倒入50 mL的异丙醇(IPA,纯度≥99.7%)中混合均匀;再将其置于超声仪中以120 W的功率超声2 h,超声后的溶液以500 r/min离心5 min,取上层清液即为FeS QDs溶液。
-
实验采用共混法制备FeS QDs/PVA纳米复合薄膜,步骤如下:分别取0.4 g PVA粉末和20 mL去离子水加入烧杯中,磁力加热搅拌45 min直至粉末完全溶解,再加入4 mL FeS QDs溶液继续加热搅拌15 min;取4 mL混合溶液滴铸于金属样品盒内;放置在40 ℃加热板上加热4 h成膜。
-
采用透射电子显微镜(TEM, FEI Tecnai G2 F30 S-Twin)、原子力显微镜(AFM,日本精工SPA-400)及能谱仪(EDS, NOVA NANOSEM 450)对FeS QDs的尺寸、形貌、结构和元素组分进行表征测试;采用X射线光电子能谱 (XPS,PHI Versa 探针 II)、傅里叶变换红外光谱(FTIR, Nicolet iS10)、X射线衍射(XRD,Empyrean Ultima Ⅳ)和拉曼光谱(Raman, Horiba Jobin-Yvon LabRAM HR800)对FeS QDs的物相组成及成键特性进行分析;采用紫外-可见分光光度计(UV-Vis, Shimadzu UV-3600)和荧光光谱仪(Hitachi, F-4500)测试FeS QDs及FeS QDs/PVA纳米复合薄膜的光学特性。
-
文中以IPA为分散剂,采用液相超声剥离法制备FeS QDs,反应机理如下:超声波在IPA溶液体系中产生高频振荡,使IPA发生膨胀,产生大量空化微气泡。随着气泡的不断生成、长大以及炸裂会产生持续且强烈的冲击波,导致FeS颗粒间强烈的相互碰撞,不断破坏FeS内部的化学键和分子结构,使FeS大颗粒逐步解体形成小颗粒,小颗粒被超声冲击后继续解体,最终形成分散性良好的FeS QDs。在超声作用下,FeS QDs具有良好的分散性和均匀性,未出现聚集。在此过程中,通过调控反应参数和超声波功率,可获得不同尺寸的FeS QDs,进而实现对其光电特性的调控。
在液相超声剥离制备中,溶剂的选择至关重要,直接影响到QDs的形成和特性。IPA是一种有机极性溶剂,文中作为超声传导介质使用。极性溶剂具有较强的溶解和分散能力,其所含的官能团(如羟基、羰基等)能与FeS表面的硫原子产生相互作用,有利于FeS QDs剥离并在溶液中保持稳定的分散态。而非极性溶剂的溶解和分散能力都较弱,不利于FeS QDs的剥离和分散。此外,溶剂黏度也会对FeS QDs的制备产生影响:黏度过高会导致超声剥离效果差,QDs分散性降低;黏度过低则会出现剥离不均匀,QDs易团聚。适宜的黏度有利于溶剂中形成微小流动,促进FeS QDs的剥离和分散。除了溶剂,FeS粉末粒度、反应体系温度等也是影响FeS QDs制备的因素,需要通过多次反复实验才能最终确定。
FeS QDs/PVA纳米复合薄膜是采用共混法制备得到,通过调控PVA溶液的浓度,可促进后期薄膜的形成,利用磁力搅拌将FeS QDs分散在PVA溶液中,可提高薄膜内QDs的均匀性。在共混的过程中通过静电吸引力、氢键和范德瓦尔斯力等相互作用,使FeS QDs和PVA结合在一起。在加热条件下,PVA分子链开始交联和固化,形成交联网络结构,FeS QDs被嵌在PVA基质中,最终形成稳定的FeS QDs/PVA纳米复合薄膜。
-
图2(a)是FeS QDs的TEM图和粒径直方分布图,可以看出QDs分散性良好,粒径分布在6~10 nm之间,平均尺寸约为8.1 nm。由于量子点的带隙大小主要与其尺寸有关,故利用公式(1)对FeS QDs的带隙进行计算[9]。

Figure 2. The morphology, structure and component characterizations of FeS QDs. (a) TEM image (inset shows the particle size distribution); (b) HR-TEM image; (c) Crystal structure model; (d) AFM image; (e) Height analysis of the particle sizes at positions 1, 2, and 3 marked in Fig.(d); (f) EDS energy spectrum
式中:Eg(QDs)为量子点带隙;Eg(bulk)为体材料带隙;R为量子点半径;me、mh及εr分别为材料的电子有效质量、空穴有效质量和相对介电常数;h为普朗克常数;e为元电荷;ε0为真空介电常数。将FeS的相关参数[10-13]代入公式(1),计算得到FeS QDs的带隙为0.23 eV。由于QDs的带隙值与其激子峰的波长成倒数关系[14],可知文中的FeS QDs在红外波段具有良好的吸收能力。
图2(b)是FeS QDs的HR-TEM图,其晶格间距为0.264 nm,对应(1, 0, 1)晶面。通过Materials Studio软件对FeS QDs建立晶体结构模型,如图2(c)所示,Fe原子和S原子沿着(1, 0, 1)晶面有序排列,形成稳定的晶体结构。图2(d)是FeS QDs的AFM表征结果,可以看到QDs尺寸均匀,分散性良好,从中随机选取三个QDs,分别标记为1、2、3,进行粒径高度分析,如图2(e)所示,高度分别约为8.5、8.4 、9.1 nm,平均高度8.7 nm,该结果与TEM测得的尺寸相近,表明制备的FeS QDs呈球形。通过图2(f)的EDS元素分析图,可以得知FeS QDs中元素的相对含量:排除铜网中的Cu、C等元素的干扰,Fe元素(53.2%)和S元素(46.8%)的原子比例近似为1∶1。
-
图3(a)是FeS QDs的XPS全谱图,图中有4种类型的峰,分别为S 2p、Fe 2p、C 1s和O 1s,其中C 1s和O 1s可能是分散剂IPA残留导致的;为了深入分析FeS QDs中S 2p和Fe 2p的存在形式,对其进行分峰拟合处理,S 2p的XPS谱如图3(b)所示,共有4种形式: S2− 2p3/2(161.42 eV)、Sn2− 2p3/2(163.16 eV)、S2− 2p1/2(164.41 eV)和SO42−(168.95 eV),其中SO42−可能是由于S2−被空气中的氧气氧化所导致;Fe 2p的XPS谱如图3(c)所示,共有4种形式:Fe2+ 2p3/2(710.48 eV)、Fe3+ 2p3/2(712.01 eV)、Fe3+ 2p1/2 (720.32 eV)和Fe2+ 2p1/2(724.73 eV),根据峰值强度进行分析Fe 2p3/2占主导,这与文献[15-17]报道的峰位位置相接近。图3(d)是FeS QDs的红外傅里叶变化光谱表征结果,*标记的两个峰从左到右分别为O-H和C=O的伸缩振动吸收峰[18],这可能是因为有机溶剂IPA中的C、H和O结合导致的,1108 cm−1 和616 cm−1处的吸收峰对应Fe-S的伸缩振动[19],表明样品中存在FeS。图3(e)是X射线衍射谱的测试结果,通过对比PDF标准卡片得知FeS晶胞中Fe-S的键长为0.217 nm,与Fang[20]报道的一致,图中2θ=30.1°、2θ=33.9°、2θ=43.6°、2θ=53.4°和2θ=71.3°处的衍射峰分别对应于FeS的(1, 0, 0)、(1, 0, 1)、(1, 0, 2)、(1, 1, 0)和(2, 0, 2)晶面,图中没有出现其他衍射峰,表明样品纯度高;图3(f)是FeS QDs的拉曼光谱图,在220.9、288.2 cm−1处均出现特征峰,分别归因于FeS的不对称和对称拉伸模式,即B1g模式和A1g模式,表明FeS是切实存在的[21-23]。
-
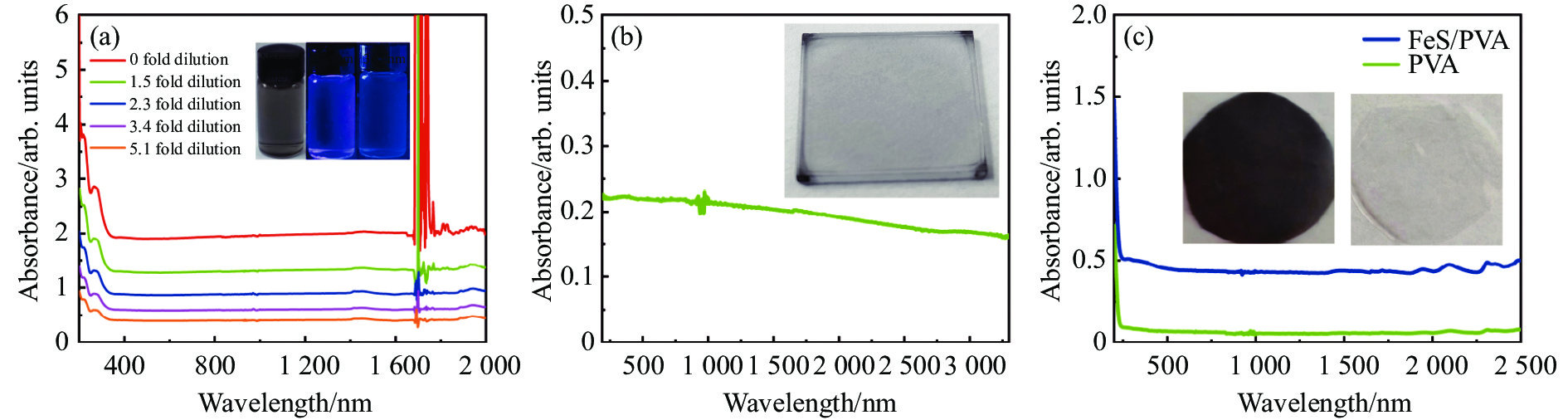
图4(a)是FeS QDs的UV-Vis吸收光谱测试结果,可以清楚地看到FeS QDs在紫外到近红外波段(200~2 000 nm)都存在较强吸收,当波长大于350 nm时,同一个样品的吸收强度不随波长增加而变化。图4(a)中的插图是FeS QDs溶液在不同光照下的照片,可以看到样品在自然光下呈灰黑色,在波长为254 nm和365 nm的紫外光照射下分别呈紫色和蓝色,表明FeS QDs溶液存在荧光效应。为进一步研究纯FeS QDs薄膜的吸收特性,将FeS QDs溶液旋涂在石英衬底上成膜进行测试,如图4(b)所示,FeS QDs薄膜在紫外波段到红外波段(200~3300 nm)都具有吸收,随着波长的增加,薄膜的吸收强度逐渐降低,但下降幅度不大。图4(c)是PVA薄膜和FeS QDs/PVA纳米复合薄膜的UV-Vis吸收光谱,插图为自然光照下拍摄的PVA薄膜(右)和FeS/PVA纳米复合薄膜(左)的照片,测试表明PVA薄膜自身在250~2500 nm波段内没有明显吸收,而FeS QDs/PVA纳米复合薄膜在200~2500 nm范围内都具有较强的吸收,且吸收强度不随波长增加而变化,表明FeS QDs与PVA复合成膜后从紫外到红外波段具有稳定的吸收。

Figure 4. UV-Vis absorption spectrum of FeS QDs and FeS QDs/PVA nanocomposite films. (a) FeS QDs solution, (inset: FeS QDs solution under natural light and UV light); (b) FeS QDs films; (c) PVA films and FeS QDs/PVA composite films (inset: photographs of PVA films (right) FeS/PVA composite films (left))
图5(a)是FeS QDs溶液的PL光谱图,随着激发光波长的增加,PL的峰值逐渐向长波方向移动,为了更直观地反映这一现象,对其进行归一化处理,结果如图5(b)所示,其峰值位置从525 nm增加到601 nm,表明PL峰对激发波长具有依赖性。这种现象可能是由量子尺寸效应和表面态引起的;由于量子尺寸效应,FeS QDs能带结构和能级分布受到限制;当激发波长增加时,FeS QDs中电子跃迁到导带的能量也会增加,这将导致激子的束缚能量增加。而PL峰的位置通常对应于激子的发射能量,因此FeS QDs的 PL峰会出现红移现象;表面态的存在可能导致FeS QDs形成多个能级,对应多个能级跃迁。综合考虑以上因素,PL峰对激发波长表现出依赖性。该现象在大部分QDs中均有体现[24-27]。图5(a)插图是FeS QDs在近红外波段的PL光谱图,由此可以看到,当激发波长从550 nm增加到700 nm时,FeS QDs在近红外波段仍存在发光现象。图5(c)是FeS QDs的PLE光谱图,当发射光波长从480 nm增加到650 nm时,FeS QDs的PLE峰也出现类似PL峰的红移现象。归一化处理的PLE光谱图如图5(d)所示,其峰值位置从441 nm增加到520 nm;图5(c)插图是FeS QDs在近红外波段的PLE光谱图,当发射光波长从600 nm增加至750 nm时,可以看到FeS QDs在近红外波段仍存在较强的响应。图5(e)为FeS QDs/PVA纳米复合薄膜的PL图,可以看出当激发光波长从280 nm逐渐增加到400 nm时,复合薄膜的PL峰同样表现出对激发波长的依赖性,图5(e)的插图是FeS QDs/PVA纳米复合薄膜在近红外波段的PL光谱图,当激发光波长从420 nm增加至500 nm时,同样可以观察到其在650~900 nm的近红外波段存在发光现象。图5(f)是将图5(e)进行归一化处理后得到的,可以看到纳米复合薄膜出现明显的红移现象。

Figure 5. PL and PLE spectra of FeS QDs and FeS QDs/PVA nanocomposite films. (a) PL plots of FeS QDs (inset: PL plots of NIR); (b) PL plots of FeS QDs normalised; (c) PLE plots of FeS QDs (inset: PLE plots in NIR); (d) PLE plots of FeS QDs normalised; (e) PL plots of FeS QDs/PVA composite films (inset: PL plots of the composite films in NIR); (f) PL plots of FeS QDs/PVA composite films normalised
-
文中以FeS粉末为前驱物,采用液相超声法制备出分散性良好的FeS QDs,平均粒径约8.1 nm,平均高度约8.7 nm,并通过共混法得到FeS/PVA纳米复合薄膜;经计算得出FeS QDs的带隙为0.23 eV,在红外波段具有良好的吸收能力。UV-Vis测试,表明FeS QDs的溶液、薄膜及FeS/PVA纳米复合薄膜在紫外到红外波段具有明显吸收;通过PL和PLE测试发现,FeS QDs和FeS/PVA纳米复合薄膜在红外波段均有光致发光特性,且随着激发波长的增加,峰位出现红移,表现出激发波长依赖的发光特性。综上所述,FeS QDs及FeS QDs/PVA纳米复合薄膜均具有良好的红外光学特性,在红外探测、生物医学和光电器件等领域具有重要的应用潜力,有望成为一种新型红外材料。
Study on preparation and infrared properties of FeS quantum dots and their composite films
doi: 10.3788/IRLA20230489
- Received Date: 2023-09-10
- Rev Recd Date: 2023-10-25
- Available Online: 2023-12-22
- Publish Date: 2023-12-22
-
Key words:
- liquid phase ultrasonic exfoliation /
- FeS /
- quantum dots /
- nanocomposite film /
- infrared characteristic
Abstract:





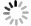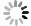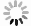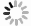






 DownLoad:
DownLoad: